2860: Decay Modes
| Decay Modes |
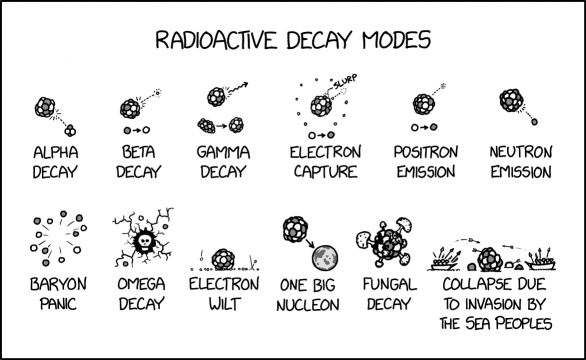 Title text: Unlike an Iron Age collapse, a Bronze Age collapse releases energy, since copper and tin are past the iron peak on the curve of binding energy. |
Explanation
| |
This explanation may be incomplete or incorrect: Created by an EXTANT MODE OF DECAY - Please change this comment when editing this page. Do NOT delete this tag too soon. If you can address this issue, please edit the page! Thanks. |
Decay modes refer to the different ways in which unstable atomic nuclei transform into more stable ones, typically by emitting particles or radiation. The process of decay is a natural phenomenon that occurs in radioactive substances. There are several types of decay modes, each characterized by the particles emitted or the energy released during the process. In the comic's diagram, protons are white and neutrons are gray.
The first six modes are real, and most occur relatively frequently:
In alpha decay, an unstable nucleus emits an alpha particle, composed of two protons and two neutrons. Alpha decay is the primary source of helium on earth, as alpha particles are helium-4 nuclei.
In beta decay (more properly beta-minus decay), a neutron-rich nucleus emits a W⁻ boson, converting one neutron into a proton — as shown in the diagram — which in turn decays into an electron (the titular beta (minus) particle) and an electron antineutrino. The diagram shows only the beta particle, which was the only thing expelled from the nucleus that could be observed directly when the types of nuclear decay were first described and enumerated.
In gamma decay, an unstable nucleus (represented by the lumpy, prolate nucleus in the diagram -- standing for a high-energy nuclear isomer) emits a high-energy photon known as a gamma-ray and settles into a stabler, lower-energy state.
In electron capture, a proton-rich atom slurps an electron from the K or L electron shell. This converts a proton into a neutron and emits an electron neutrino. No 'slurp' sound is actually produced in real electron capture event.[citation needed]
In positron emission, or beta plus decay, a proton-rich nucleus emits a W+ boson, converting one proton into a neutron, which in turn decays into a positron, the beta plus particle, and an electron neutrino. Again, the diagram shows only the beta particle, presumably for simplicity. This is much rarer than beta minus decay.
In neutron emission, a neutron-rich/proton-deficient unstable nucleus emits a neutron (which then goes on to decay into further daughter particles).
The other six modes are fictional:
Baryon panic: In this mode, all the subatomic particles flee the atom simultaneously, similar to a crowd fleeing a building during a fire alarm, or other similar states of panic in people. In reality, this mode of decay would require an incredible amount of energy. The like charges of protons do repel each other, but they are held together more tightly by the residual nuclear force in the presence of neutrons.
Omega decay: The atom has decayed and left behind a skull in its wake, leaving cracks in the area surrounding it and send neutrons and protons flying everywhere. Whereas alpha, beta, gamma are the first three letters of the Greek alphabet, omega is the last, so the name omega might suggest the ultimate, final decay. The skull presumably represents the finality of such a decay, given that the end stage of human decay leaves behind a skeleton, something that does not exist in nucleons.[citation needed] Many works of science fiction propose forms of radiation and/or particles with further letters in the Greek alphabet, such as The Omega Directive in Star Trek. In real life, the omega baryon was predicted to exist by Murray Gell-Mann's early quark theory, and then discovered several years later with the properties he had predicted.
Electron wilt: The electrons surrounding the atom fall to the ground. Some plants are subject to diseases that cause this kind of wilting of their leaves. Electrons will attempt to settle into a 'ground state' but this does not involve them literally slumping to the ground, rather they will be as close as possible to the nucleus subject to the limitations of energy levels and the Pauli exclusion principle.
One big nucleon: The protons and neutrons combine to form a single huge baryon. Exotic baryons with more than the usual three quarks, such as pentaquarks, have been created in the lab but are not known to exist in nature. String theorists propose that black holes are actually fuzzballs, single "subatomic" particles which are macroscopic in size (namely that of their event horizon) formed by the fusion of the strings of in-falling matter under extreme gravitational conditions.
Fungal decay: The nucleus rots, and fungal fruiting bodies (toadstools and mushrooms) grow around it. This plays on the meaning of "decay".
Collapse due to invasion by the Sea Peoples: The atom floats in water, with boats on either side full of Cueballs shooting arrows at it, and the atom is breaking up. The Sea Peoples are a somewhat mysterious group that attacked Egypt in the late Bronze Age (1200-900 BCE) and are associated with a widespread societal collapse around the central and eastern Mediterranean.
Transcript
| |
This transcript is incomplete. Please help editing it! Thanks. |
Discussion
Omega Decay has a didtinctive Star Trek Voyager vibe, I believe... ;-) https://memory-alpha.fandom.com/wiki/Omega_molecule 162.158.203.70 23:03, 27 November 2023 (UTC)
- There are a few things Omega could relate to: Rick and Morty Omega Device https://rickandmorty.fandom.com/wiki/Omega_Device, Galaxy Quest Omega 13 Device https://galaxyquest.fandom.com/wiki/The_Omega_13_Device 172.68.126.134 02:46, 28 November 2023 (UTC)
- Omega voyager vibe? Nah, Voyager just used a cool sounding name. They share a root, but this isn't depending on ST:VOY 172.69.195.47 09:09, 28 November 2023 (UTC)
There appears to be an issue- the fungal decay and sea peoples are missing. I don't remember what they were! Help! 162.158.159.226 23:55, 27 November 2023 (UTC)Fizzgigg
"One big nucleon" looks a lot like a planet to me.Nitpicking (talk) 03:02, 28 November 2023 (UTC)
I was rather hoping that bismuth would appear as a product, even if entirely unintentional, but it's far too high up the chain to ever occur from "bronze decay"... 172.70.85.147 14:01, 28 November 2023 (UTC)
Protons shown in white, while the neutrons in black in the comic. Nothing wrong with this but if you visualize it the other way it makes this very confusing. 162.158.62.120 (talk) 19:11, 28 November 2023 (please sign your comments with ~~~~)
The transcript might need some rearranging, because the labels are technically under the diagram? although that might make it confusing. or less confusing.--Mushrooms (talk) 18:01, 29 November 2023 (UTC)
Part of the explanation for alpha decay seems a bit mixed up: "...proton-rich / neutron-deficient heavy nuclei, which normally have many more neutrons than protons." Surely 'proton-rich' means more protons and 'neutron-deficient' means fewer neutrons, so such a nucleus would have many more protons than neutrons, wouldn't it? I hesitate to change the explanation because I'm more of a language expert than particle physicist. 172.68.64.226 00:26, 3 December 2023 (UTC)
- Consider uranium 238, which has 92 protons and 146 neutrons. It decays by alpha radiation to thorium 234: 90 protons and 144 neutrons. In both cases, there are a lot more neutrons than protons, but the ratio of neutrons to protons is higher in the latter because if N > P, N/P < (N-2)/(P-2). Or polonium 210, with 84 protons and 126 neutrons, which decays by alpha (as the last step in the U-238 decay series) to stable lead 206, with 82 protons and 124 neutrons. With sufficient decrease in the number of protons and increase in the N/P ratio, the system becomes stable. All elements have multiple possible isotopes, and as the proton count increases, the number of neutrons needed for stability tends to increase a bit more quickly. If there aren't quite enough neutrons, a common decay mode is alpha, which decreases the proton count and "improves" the ratio. If the number of neutrons is a bit too high for stability, the most common decay mode is beta, increasing the number of protons and decreasing the number of neutrons, again "improving" the ratio. This is a gross oversimplification, of course. BunsenH (talk) 05:44, 3 December 2023 (UTC)
- I read "...proton-rich / neutron-deficient heavy nuclei, which normally have many more neutrons than protons." as "This example has more protons and less neutrons than you'd expect for a nucleus of this weight. One with this many nucleons, in total, should consist of a greater proportion of neutrons"... But it does look a bit confusing. Definitely would be open to a rewrite (but not flipping the beginning, which'd only be rightly understood when wrongly comprehended, and vice-versa). 172.70.85.163 13:41, 3 December 2023 (UTC)
