2214: Chemistry Nobel
| Chemistry Nobel |
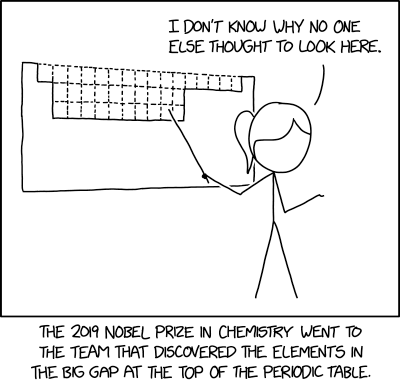 Title text: Most chemists thought the lanthanides and actinides could be inserted in the sixth and seventh rows, but no, they're just floating down at the bottom with lots more undiscovered elements all around them. |
Explanation[edit]
The periodic table of the elements is a display which arranges all of the 118 (currently) known chemical elements by atomic number and sorts them into columns such that each column contains a group of elements displaying similar chemical properties. The original version of this table was developed by Russian chemist Dmitri Mendeleev in 1869, when he realized that certain properties repeated periodically as elements became more massive. Notably, this system left obvious gaps at the top of the table. Mendeleev correctly predicted that some of these gaps represented elements that had not been discovered yet, and even predicted their properties based on the patterns in the table. The later discovery of those elements (including germanium and gallium) helped validate Mendeleev's work. Other gaps, however, were not due to undiscovered elements, but merely resulted from the properties of electron orbitals in atoms: upper rows of the table represent orbitals with fewer possible electrons and hence fewer elements, so displaying the lower rows properly below the upper ones leaves gaps in the upper rows. In other words, elements could not actually exist in these spaces, spaces which only existed in the realm of human bookkeeping. The joke of this comic is that it treats these gaps as if they represented elements that hadn't been discovered yet. Ponytail and her team have won the Nobel Prize in Chemistry merely by looking for and finding these elements. She expresses surprise that no one else had thought of such a simple direction for research.
By definition, each element has one more proton than the previous element - so element 1, hydrogen, has one proton in the nucleus, while element 2, helium, has two protons in the nucleus. The periodic table represents elements in their atomic form, where there are an equal number of protons and electrons (as opposed to an ionized form where they are unequal), so the structure of the periodic table is based on the structure of the "orbitals" that electrons fall into.
The first row of the periodic table has elements whose electrons only have an "s orbital" (at least when the electrons are in their ground state, which is the non-excited state that they are normally in). There is only one s orbital in each row, and an s orbital only has room for two electrons, so there are only two elements in the first row. The Pauli exclusion principle, mentioned in xkcd 658, means that only two electrons can be in each orbital. The second row of the periodic table contains elements with only s and p orbitals. As mentioned, there is only one s orbital at each "level" of orbital, with each level basically corresponding to a row, but there are three p orbitals at each level, so there can be four total pairs of elements in the second row, for eight total elements in the second row. (You can see that level one has a total of 1^2 orbitals, or 1 orbital, while level two has 2^2 orbitals, or 4 orbitals.) After p orbitals, the next type of orbital that can exist at higher levels is a d orbital. For levels that have a d orbital, there are five d orbitals at each level. Beginning with the fourth row, you can see elements whose highest-energy electrons are in an s orbital (the first two columns), a p orbital (the last six columns), or a d orbital (the middle ten columns). The d orbitals for row four are actually classified as the 3d orbitals (meaning they belong to level three), but because they have higher energy than the 4s orbital, they are put on the fourth row. The "aufbau principle" says that electrons fill the lowest energy orbitals first, which means that level one orbitals get filled before level two orbitals, which get filled before level three orbitals, and that within each level the s orbitals get filled before the p orbitals. So, there are two columns on the periodic table for each orbital - although helium is put in the far right instead of in the second row with the other elements whose highest electron is the second one in an s orbital, because putting it on the far right shows that helium is stable like the other "noble gases" in the far right row.
The final type of orbital that exists as the ground state for a known element is the f orbital, but almost all periodic tables show the elements with their highest electrons in an f orbital - the lanthanides and actinides that are mentioned in the title text and described below - in rows below the table, to prevent the table from becoming too wide to print easily.
The comic is based on the joke that somehow every physicist and chemist for generations somehow missed that there are actually p and d orbitals at levels one and two, and so it shows the empty space in the columns corresponding to the p and d orbitals in level one and the d orbitals in level two being filled with undiscovered elements. In reality, there are no p or d orbitals at the first level and no d orbital at the second level, due to quantum mechanics (involving the possible values of something called the quantum n, l, m, and s numbers, where n is the level and l determines whether is an s, p, d, or f orbital). The comic also shows a line of d orbital elements in the third row, even though the 3d orbitals are already represented in the fourth row (where they are placed due to having higher energy than the level 4 s orbitals). The Pauli exclusion principle has been known since 1925, and Mendeleev (mentioned in xkcd 965) developed the structure of the periodic table in 1863 to describe the structure of the known elements, so the idea that such a basic thing as more elements in the early rows that had never been discovered by any chemist ever would be quite surprising. In reality, the elements toward the top of the periodic table that are known to be naturally occurring were generally discovered earlier, while all the most recently discovered elements are higher-numbered elements lower down on the table that are very short-lived before they undergo radioactive decay to another element and have never been seen to be naturally occurring.
The lanthanides and actinides mentioned in the title text are series of elements with higher atomic numbers that have electrons in orbitals that no previous elements have, and thus occupy columns of the periodic table that don't exist for lower-numbered elements. Sometimes these elements are displayed in the table, a format that corresponds with their actual orbital structure; this format is too wide for most display media, thus the lanthanides and actinides are separated out and displayed "floating" beneath the rest of the periodic table. The title text jokes that these floating series of elements are actually surrounded by actual elements.
In real life, the 2019 Nobel Prize in Chemistry was awarded to John B. Goodenough, M. Stanley Whittingham, and Akira Yoshino for their work in the development of lithium-ion batteries; it was announced on October 9, just a few days before this comic was published, so the chemistry Nobel Prize was in the news.
Transcript[edit]
- [Ponytail stands in front of an image with a white section in the shape of the 7 rows of the periodic table of the elements, but without the two rows usually shown beneath with the lanthanides and actinides. The “empty” sections at the top of the table are filled with three rows of dotted boxes, 16 boxes in the top row and two rows with 10 boxes each, shifted one right from the top row. Ponytail points to this area with a pointer while she looks and gestures towards an off-panel audience.]
- Ponytail: I don't know why no one else thought to look here.
- [Caption below the panel]:
- The 2019 Nobel Prize in Chemistry went to the team that discovered the elements in the big gap at the top of the periodic table.
Discussion
No Discussion yet? REALLY?!!? 162.158.214.82 15:23, 12 October 2019 (UTC)
This may be a reference to SCP-2046. 162.158.146.34 15:40, 12 October 2019 (UTC)
- Or something else. From the beginning, what are the ten radical isotopes? -- Hkmaly (talk) 21:36, 12 October 2019 (UTC)
- From the beginning, the ten radical isotopes are: Tukerium, negative 5. Dangor, negative 17. Lu, negative 31. Kartex, negative 79. Sharbar, negative 101. Muilamium, negative 127. Idaron, negative 173. Simmondsium, negative 211. Mattite, negative 239. Krasnov, negative 307. These are the radical isotopes from the beginning. --[REDACTED], 10/25/2024 04:26
Couldn't this potentially involve exotic isotopes of hydrogen that behave similarly to elements in the same group? --162.158.214.136 16:02, 12 October 2019 (UTC)
Oh gods, I needed this laugh. Have my Chemistry exam on Monday, this does put a smile on my face.
"misconception that the empty space at the top of the periodic table represents undiscovered elements"... [citation needed]. Is that really a thing? Never heard of it. Ralfoide (talk) 16:53, 12 October 2019 (UTC)
- Somehow I did not think about that the entire time I was editing this thing, because I don’t believe it is. I guess I’ll fix it. 172.69.34.56 18:32, 12 October 2019 (UTC)
Some uninvited pedantry (unlike all my other didactic discourse here, which you guys bring on yourselves): Referenced in the comic is not THE periodic table, just a periodic table. And it isn't really objectively scientific. It's better to call it the most popular periodic table. Such tables are a rather ham-handed attempt to explain the patterns of the elements in an "intuitive" (or at least heuristic) way. But the popular one we learn in school is actually far from the best one even in that sense. Check out the alternatives, many of which are more scientifically sound and logical...but aren't as simplistic for the easy-minded, so they haven't caught on. —Kazvorpal (talk) 23:37, 12 October 2019 (UTC)
- Do you mean the one that looks like a candyland board game (Benfey's) or the one that looks like the worst Tetris level ever (Tsimmerman's)? [j/k]... If I had seen that in school, I'd have been too distracted to ever pay attention ;-) Ralfoide (talk) 07:35, 13 October 2019 (UTC)
- A very interesting link. Thanks! Yosei (talk) 12:41, 2 December 2019 (UTC)
Did Mendeleev really design his table to represent the way electrons are arranged in atoms? In 1869, he must have been quite a visionary! Zetfr 09:23, 13 October 2019 (UTC)
- Oh no, he didn't. He did by patterns of their properties. Also by atomic weights, but those were imprecisely known then, also note the isotope paradox problem (e.g. K and Ar must be swapped). The first sorting already guarantess to represent the electronic arrangement to some degree. BTW, lanthanides and actinides need more love. For starters, I PhD'ed on them.
- Actually he was quite the visionary, considering what they didn't know back then. While everybody else was arranging their tables (and there were plenty of them) entirely by atomic weight, he arranged them by both atomic weight on the large scale and chemical valence on the small scale. This clued him in to the changing periods and also enabled him to correct elements out of order by weight. The noble gases hadn't been discovered yet, but when they were, they fit right in as they had a valence of zero. A few decades later Henry Mosely used proton bombardment and X-Ray radiation measurement to determine the electrostatic properties of various elements and found a simple progression that both absolutely vindicated Mendeleev and introduced the concept of Atomic Number. He should have gotten a Nobel prize, but sadly, no prizes were awarded that year because of the war and Mosely himself was killed at the young age of 27 by a bullet with his name on it. Sigh.
172.69.55.22 15:20, 13 October 2019 (UTC)
Clearly these new elements are fractional elements, with elements having - for instance - 1 3/16 protons, etc. 108.162.241.248 21:20, 13 October 2019 (UTC)
Of course, if someone did find a whole bunch of elements there, I'd say that they deserve a Nobel prize. 172.69.63.133 12:37, 14 October 2019 (UTC)
for me, the explanation provided doesn't seem to emphasize why the joke works well enough. shouldn't the explanation more clearly state that the gap between hydrogen and helium is there because the table is grouped based on blocks of elements and electron orbits. the first row only has electrons in the s orbital and none in p, d or f orbitals, and that gaps between hydrogen and helium, for example, could not possibly be filled because there isn’t anything to fill them with. similarly for the 2nd and 3rd row "gaps". this impossibility really begets the humor of a figure pointing at the gap musing "i don't know why no one else thought to look here".
In Russian books on chemistry, elements are numbered, ordered in the same way, yet the table itself is arranged in a different manner: in R20, RO, R2O3, RO2, R2O5, RO3, R2O7, RO4 way. It, however, is done to make both the table + all the extra data on each element rectangular (so it would fit into one A4 sheet).172.68.11.67 05:14, 20 March 2021 (UTC)
A girl in my high school chemistry class seriously thought this. She was trying to argue with the teacher that "There's infinitely many elements, we just haven't discovered them yet. You can't prove 1p and 2d orbitals don't exist just because we haven't seen them." Ironically she was the "religion is the cause of all society's problems" type atheists.
StapleFreeBatteries (talk) 21:45, 5 August 2024 (UTC)
- To be half fair, to her statement (as reported), there's indeed no reason to believe that there aren't an infinite number of elements, just by extending the current table down infinitely (with or without filling in the 'gaps' in the top of it, which add only a finite extra number). Beyond (well beyond?) the lanthinide/actinide full-width it doesn't even need additional shells (unseen, thus not currently featured in the 'wider gap' model, just like La/Ac groups and transitional metals crowbar the upper bits apart, and indeed everything not Group1 or Group8/0 levers H and He apart), but that might happen too – without needing the new (upper) gap to be filled as well. 172.70.91.139 14:57, 6 August 2024 (UTC)
 Add comment
Add comment
