2530: Clinical Trials
| Clinical Trials |
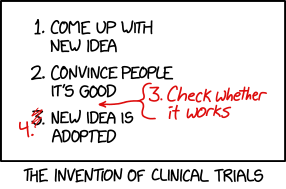 Title text: We don't need to do a clinical trial of this change because the standard of care is to adopt new ideas without doing clinical trials. |
Explanation[edit]
The comic begins with a simple process for adopting a new idea just by convincing people that it is a good idea. The joke is that this skips the important step of checking whether it actually is a good idea. That correction presumably comes about after ideas are adopted which sounded good but turn out to be harmful. The comic captions the addition of this checking step as "the invention of clinical trials".
The purpose of clinical trials in medicine is to make sure that a new medicine works and doesn't have serious side-effects. One example of the dangers of failing to make sure that it doesn't have serious side effects is thalidomide, which caused a lot of birth defects. In a clinical trial, the effect of a treatment is compared to the effect of a placebo, or an existing treatment, to make sure it actually has a beneficial effect. (Earlier trials establish that it is even a viable candidate for testing and establishing possible dosages/regimens that can then be carried forward to a treatment (Phase III) trial.)
Before the invention of clinical trials, people generally didn't know, or at least had no way of confirming, whether medicines actually worked. Although many herbs and medicines were effective, others were no better than a placebo, and some medical treatments such as trepanation and bloodletting not only had no benefit (except for a very few rare conditions) but were very likely to be harmful. Those treatments that did work at all were mostly those that had been tried (for whatever reason) and just happened to be useful, but others had neutral or even adverse effects, but still managed to not be so dangerous that subsequent recoveries from the original ailment—regardless of (or despite!) dangers inherent in such treatments—were taken as proof of their efficacy.
Similar to more recent examples, some earlier treatments may have been gradually discovered to help a particular condition only by noticing beneficial side-effects when consumed for sustenance or for unrelated medical 'guesses'. However, they also remained without the full scientific rigour so long as it remained a 'traditional remedy' with at best an oral tradition across many disparate practitioners, and no consistent effort to formalise or test the falsifiability of any findings.
At the time that this comic was published, the world was in the middle of the COVID-19 pandemic, which made the existence of clinical trials more relevant to the public, who waited eagerly for what sounded like good ideas to get through clinical trials and available to the general public… or fail clinical trials and not do that. During this frustrating wait, many unscientific claims have been made that various drugs or non-drug treatments are cures for COVID-19, making it difficult to convince believers to get real treatments. On the other hand, many people were skeptical about COVID-19 vaccines which were made available to the public for emergency use before the clinical trials were finished, or had concerns about whether the clinical trials were rushed or otherwise flawed due to how quickly they were conducted compared to the traditional speed for vaccine development and approval.
In the title text, "Standard of care" refers to the previously accepted practice which a new medicine needs to be compared against. Because the original 3-step "standard of care" in this comic didn't include clinical trials before their adoption, we didn't need to do any testing in order to decide to start using them. If we had had them as the standard of care, then we would have had to perform tests before we added a step and it would have taken longer. This assumes that the process itself is subject to the same scientific rigor as medical treatment; in practice that would be more of a political change that is still not tested.
Transcript[edit]
- 1. Come up with new idea
- 2. Convince people it's good
- [Scrawled in red as an afterthought, an arrow inserting it between item 2 and the original item 3]
- 3. Check whether it works
- 3. [Now scribbled over and amended to "4."]
- New idea is adopted
- [Caption below the panel]
- The invention of clinical trials
Discussion
Is this comic in reaction to some specific recent event? It seems like it might be related to vaccine trials, given the pandemic the world has been dealing with for the last 2 years... if so, it then seems to be a condemnation... am I reading too much into this? Ericfromabeno (talk) 21:49, 18 October 2021 (UTC)
- That's exactly how I understood it as well. Maybe that wasn't Randall's intent, but it does seem to convey a skeptical tone about the untested vaccines and their related mandates? 127.0.0.0 18:56, 19 October 2021 (UTC)
- Except the vaccines HAVE gone through clinical trials? Calling the vaccines "untested" is quite a reach, since they have actually been very well tested. 172.68.132.228 18:56, 22 October 2021 (UTC)
- They may have been through smaller, short-term trials, but we don't have any long-term effects data. Essentially, everyone who has gotten a shot is in one big trial.--108.162.237.243 00:44, 5 November 2021 (UTC)
- Yeah, a trial consisting of 3.3 billion fully vaccinated people. If it was any worse than being unvaccinated, wouldn't we have noticed by now? 256.256.256.256 (talk) 07:41, 6 December 2021 (UTC)
- They may have been through smaller, short-term trials, but we don't have any long-term effects data. Essentially, everyone who has gotten a shot is in one big trial.--108.162.237.243 00:44, 5 November 2021 (UTC)
- Except the vaccines HAVE gone through clinical trials? Calling the vaccines "untested" is quite a reach, since they have actually been very well tested. 172.68.132.228 18:56, 22 October 2021 (UTC)
I would say this in relation to the mutiple treatments for Covid19 some of which have great clinical evaluation, others less so. I'll make a first draft Kev (talk) 21:53, 18 October 2021 (UTC)
- On this website there are far too many people who think everything is about Covid19. --162.158.88.219 06:30, 19 October 2021 (UTC)
- It comes to mind after a year full of iterations of the "3-step clinical trial procedure" shown in the comic. See e.g. https://en.wikipedia.org/wiki/List_of_unproven_methods_against_COVID-19
Note that a proper clinical trial does not "prove" its treatment to be effective, but it actually should do its damnedest to show that any observed (net) benefits are down to simple statistical fluke, but then fail, leaving the positive result 'proven'. And obviously extract every possible risk factor in the process. (Thalidomide fell down badly on this, many years ago, partly because of the numbers involved and the fact that susceptible mothers were often taking a cocktail of multiple 'remedies' over much of the nine months, which made the reality slow to be teased out. But the lessons learnt mean that authorising anything for pregnant women are tortuous, and testing on (non-pregnant) women in general is hampered by having to account for menstral cycles, so we end up with far too many man-tested drugs that say "not for use in pregnancy" just to keep far to the safe-side, plus still far more unknown levels of efficacy/etc in the 'generic' female body than we should have. But it's being addressed. Onward, ever onward!) 162.158.159.49 23:14, 18 October 2021 (UTC)
The way I understood the title text was that the "change" was the one written in red, that is "now we have to do clinical trials". The title text's joke is thus that, because before that change clinical trials weren't part of the procedure ("standard of care"), you don't have to test the idea of testing ideas. Closely related to that "joke" is https://existentialcomics.com/comic/404 (but seriously this isn't a simple problem). 108.162.229.101 01:21, 19 October 2021 (UTC)
Shouldn't Test if it works be step 2? Have idea, see if it works, impliment? 172.68.129.137 01:52, 19 October 2021 (UTC)
- No. Steps 1 and 2 both include elements of testing and exploration; you need to perform experiments to come up with a good idea and convince -yourselves- that it is, in fact, good, and then you likely need to perform or at least show more tests to convince others that it is, in fact a good idea. But the addition of clinical trials added a further "and then you need to double triple check that your idea actually works rather than that it seemed to work in your initial experiments" step to (try to) avoid bad side effects and false correlation.
- I'm of the opinion that it was part of the joke... since it does seem to follow the actual behavioral pattern of "do thing, promote thing, [justify thing], propagate thing"... which makes this thread of conversation both topical and meta (kudos to Randall if this result was intended)162.158.107.4 21:14, 19 October 2021 (UTC)
- I'm guessing you've never written a grant proposal. To get money to test something, you have to convince people it's worth testing. 172.68.133.103 19:01, 22 October 2021 (UTC)
Mention of "anti-worming treatments" in the explanation. This is misleading, and gives the impression that drugs can only have a single function. It's like talking about the use of "headache medicine" for preventing heart attacks. If you want to refer to a specific medicine, do so by name but make damn sure that your claims about that medicine are accurate
- Ivermectin, the drug in question, has only been approved for use by humans for parasites, and for rosacea (cause unknown, but appears to be linked to mites). It doesn't seem misleading to me to refer to it as a deworming agent when it is probably only ever prescribed as an anti-parasitic. 172.68.132.16 19:18, 22 October 2021 (UTC)
While the awareness of clinical trials is of course more relevant because of COVID, I don't think this is intended to be topical. The title is very straightforwards-- "the invention of clinical trials" and is almost joke-less (basically just the format). The real joke is in the title text, where it's pointed out that because the "standard of care" before the invention of clinical trials was not to do clinical trials, we didn't need to go through this step to start doing them; just convince people it was a good idea. 02:40, 19 October 2021 (UTC)
- I think it's *extremely* topical, with the relatively recent debunking of ivermectin as (yet another) substance that has been widely claimed, distributed, and mis-used as a supposed COVID preventative/cure. BunsenH (talk) 03:49, 19 October 2021 (UTC)
A recent editor pulled out my comment about how there isn't a joke, but I'd argue that that's necessary in some form. One of the reasons people go to Explain XKCD is that they're going "wait, did I miss a joke?" So explaining that as far as the community is concerned the main text is in-earnest education rathar than a missed joke does have an important purpose. Mneme (talk) 04:37, 19 October 2021 (UTC)
This list looks like the difference between philosophy, particularly ancient Greek and Indian philosophy (probably others, but I do not know them as well), and science. The philosophical ideas were adopted based on who was able to convince more people that they had a better idea. When the scientific revolution rolled around in the 15th and 16th century in Europe, many of these ancient ideas were actually tested and only those that really worked were retained as true. Many well known and well respected ideas that failed testing were finally abandoned. That sure sounds like the elements of this list. Nutster (talk) 07:11, 19 October 2021 (UTC)
The Covid-19 paragraph seems a bit misleading. Arguments advanced in favour of vaccine skepticism have mostly been not so much to do with treatments being used before clinical trials were complete, as with clinical trials being brought forward to accelerate the process, which has been misinterpreted as them being 'rushed'. 162.158.154.206 10:38, 20 October 2021 (UTC)
Traditional clinical trials aren't the only way to find out whether a particular medical treatment works; a lot of treatments used for various purposes instead get tested and adopted by comparing the outcomes of the treated group to prior outcomes of people with the same condition, or simply comparing whether the healths of a group of people improve after treatment with a given treatment with a known mechanism of action (thus circumventing the correlation/causation disconnect); for ethical reasons, not everything can be put through traditional clinical trials (as it would be unethical to deny a patient [even one who's part of a control group] treatment that's been shown to have lifesaving effects in other patients), and is thus used technically-off-label based on these secondary ways of showing efficacy (for instance, gender-affirming hormonal and surgical treatment for transgender individuals). Whoop whoop pull up (talk) 17:58, 26 March 2024 (UTC)
- I'd argue that "traditional clinical trials" were pretty much all "we gave our tincture to everyone, and they almost all survived". It's the lack of rigour that introduced various additional elements, like the double-blind New Treatment vs. Old Treatment, or Treatment vs. Placebo, 'golden standard'.
- CTs cover "investigational" (just look how people are doing/have done under whatever course of treatment they might have, with no actual provision of anything new) and "interventional" (as above). A good study design will have a way to check the ongoing treatment (still blind/anonymised) and, if it warrants it (and it's not a review of historic data!), breaking the double-blind early and offering all subcohorts any treatment (or 'default') that has outperformed the other(s) being given. Useful both if the investigated drug is shown to be a 'miracle cure' or if it's a flop/worse than the old drug/has too many additional adverse events to be considered safe.
- A modern trial (well... a decade ago, when I was work-adjacent to such trials, so picked up a lot from my colleagues who actually did trial design and management) probably covers all the points you suggest, all still under the same umbrella of "Clinical Trial". The exact specifics of how they proceded would depend a lot on what the trial was for, etc, and by the time Phase III (full clinical trials) were being started, the results of the Phase I/II (first-in-human/sub-theraputic tests) would have been concluded or wound up early (with sufficient success), giving a good idea of how much 'denied treatment' (or "given the prior treatment") is absolutely required, without being potentially excessive in caution.
- And pretty much every one is bespoke. Trials of off-label uses, extending trials of adult-approved drugs to adolescents/children, or allowing their previously barred use in pregnant women. Restudying a drug that hadn't been tested at all in women (allowing for pesky hormone cycles, perhaps, which the men-only test had avoided). Intensively studying something truly life-saving (resuscitation methods, anti-AIDS treatments, pandemic vaccines) or quality-of-life extending (anti-dementia, treatments for Parkinsons, less traumatic chemotherapy). - Some, if not all, will consider how quickly the generously volunteering (or 'volunteered') control group might still be able to benefit from their originally being not-fully-treated (where possible, practical and proven as advantageous).
- But it's not something I was directly involved in, my responsibilities were in making sure that those with the more direct responsibilities could do their work properly, however it was they needed to in any given instance. I think I'd have been more stressed designing and managing a trial than I was (technically could affect numerous trials, or at least potentially increasing everyone else's stress values if I totally messed). I'm sure there's a more accurate/succinct explanation available. 172.70.160.166 19:37, 26 March 2024 (UTC)
 Add comment
Add comment
