2639: Periodic Table Changes
| Periodic Table Changes |
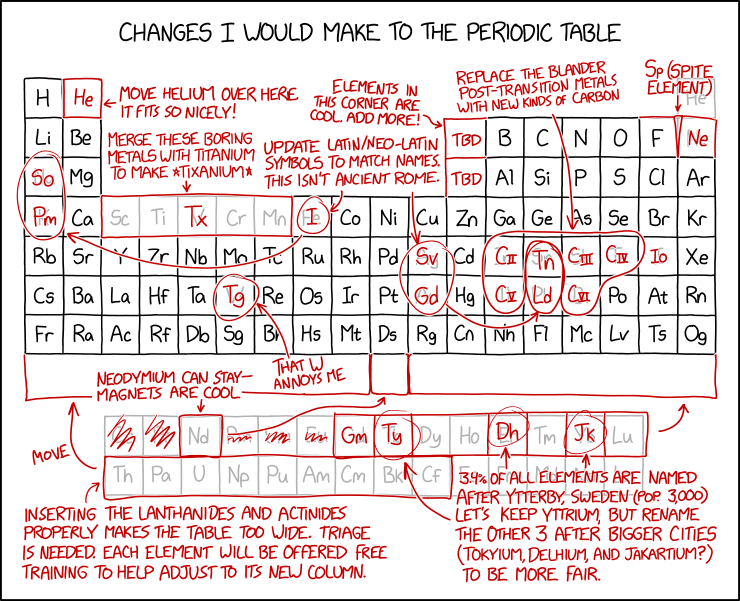 Title text: It's nice how the end of the periodic table is flush with the edge these days, so I think we should agree no one should find any new elements after #118 unless they discover a whole row at once. |
Explanation[edit]
The periodic table is a table used to arrange chemical elements according to their chemical and physical properties. This comic proposes "changes" to the periodic table that would be more pleasant aesthetically or make the periodic table look more regular. Some of these are (somewhat) practical changes to element abbreviations that could improve clarity in English, though changing documents to use different abbreviations would probably be more trouble than it's worth. However, other changes move elements around without considering that elements would stop being arranged by their properties. The periodic table would stop being useful after such changes unless said changes were meant to physically change the material properties of the elements, which would be impossible, although the comic plans to solve the problem with "free training" to their atomic behavior.
Other modifications make up new elements or remove existing ones from the table, which would not be a reasonable decision given that the periodic table is supposed to include all existing elements, whether they make the table neater or they don't. See explanation for the proposed changes in the table below.
The title text suggests discovering elements only in entire rows at once, a suggestion which is far more unreasonable than it appears on its surface. Elements with more protons than 118 could be discovered in future by collisions in particle accelerators, but as of right now, the chances of finding or synthesizing samples of such elements lasting longer than 10 quadrillionths (10−14) of a second is looking rather grim. Furthermore, the theoretical 8th period is expected to contain 50, more than any row in the current 7-period table. Taking this all into account, discovering an entire row's worth of elements all at once is nothing short of a herculean undertaking, and its difficulty only grows in orders of magnitude for each successive row. With all that said, however, elements beyond 118 are unlikely to have practical applications in science and engineering due to their vanishingly short lifespan and may probably only ever serve as intellectual curiosities. Thus, refraining from updating the periodic table prior to the discovery of the entire 8th period is not a terrible sacrifice to make in the name of preserving the table's visual aesthetics (provided data of new elements are recorded and catalogued in other ways).
This is another comic containing red annotations over a complex and established structure. It suggests changing the periodic table, which was also discussed in 2214: Chemistry Nobel.
Table of the proposed changes[edit]
| Proposed change | Explanation |
|---|---|
| Move helium over here. It fits so nicely! | Helium is moved from the upper right corner to the second column next to hydrogen. However, the reason it is placed at the far-right Group 18 and not Group 2 is because it is a noble gas, rather than a reactive alkaline earth metal. You could say helium is in group 2 because it has two electrons in its outer shell, but normal periodic tables place it in group 18, the noble gases, with which it has far more in common. Hydrogen has similar problems being in group 1, as it's a non-metal and the elements below it are metals which don't have much in common with it chemically. There are periodic tables that show hydrogen floating above the periodic table for that reason.
Group 18 was not known at the time of the original table, which used I-VII (1-7) for the otherwise full-height columns, which turns out to reflect the number of free ('valence') electrons in their outer 'orbit', in one useful understanding of the atomic model. When added, the new final column was called either VIII (having a full outer shell) or Group 0 (having none free) and placed to the right of VII. It was originally thought that these so-called "noble gases" (no relation to Alfred Nobel of explosives and science prize fame) were unable to react with other elements. However, xenon compounds were discovered in 1962, and many other noble gas compounds have been discovered since then under a variety of conditions. From the fourth row downwards the inserted block of Transition Metals represent a larger electron shell with more positions for free electrons and those elements to the right would have more electrons than those above them, by this measure, but their physical properties are still best dictated and aligned by the complement to the valence (i.e. the gap-size). The current numbering system shows the outer-shell electrons accurately in the rows where the ten new columns are, but the upper rows of columns 13-18 have ten more (or sixteen more, for Helium) than is the case. Similarly, once lanthanides and actinides are considered, the group number and outer-shell count becomes disconnected again in the opposite way. But it still seems useful enough to currently label in this manner under current IUPAC guidance. |
| Elements in this corner are cool. Add more! | TBD (to be determined). Elements in that corner, such as carbon, oxygen, phosphorous, and nitrogen, participate in covalent bonding and are the primary elements involved in biochemical reactions, which may be why they are considered cooler than other elements. |
| Sp (Spite element) | Wedged between fluorine and neon. This is a reference to spite houses, houses jammed into a narrow space to block other construction, or spite fences, which are fences built to annoy neighbors. However, such an element would have an atomic number greater than fluorine's (9) but less than neon's (10). |
| Merge these boring metals with titanium to make *tixanium* | Tixanium (Tx) replaces five metals, including titanium (Ti).
While titanium certainly has an impressive name, and is used in the aerospace industry and other high-performance applications, the others are hardly boring; manganese, for example, was part of the cover story for the top-secret Project Azorian. |
Update Latin/Neo-Latin symbols to match names. This isn't ancient Rome.
|
Since I is already used for Iodine, it gets a new abbreviation Io, and Gadolinium is re-abbreviated to Gm to free up Gd.
Note that most of these changes will actually make the table less readable if one considers languages other than English. For example, in European languages, 'I' for iron will work for Irish (Iarann) (but not for Dutch, as 'ijzer' doesn't really start with 'i' but with 'ij'. It would be capitalized, e.g. at the start of a sentence, as 'IJzer'.) and also Tamil (இரும்பு [irumpu]), while 'Fe' currently matches in French, Italian, Portuguese, and most of the languages in Spain. Similarly, Natrium is still used in most Germanic languages. This group of changes doesn't include antimony (Sb -- stibium), but that’s because it gets replaced by “carbon III” (see below). Nor is copper changed from its "Cu" for "cuprum". |
| Replace the blander post-transition metals with new kinds of carbon |
Carbon can make four covalent bonds, which means it can form a huge range of chemicals, above all ones vital to life. The post-transition metals don't have this level of interest. If there were more elements like carbon, it could allow more exciting chemistry and perhaps new kinds of life. |
| That W annoys me | Tungsten: W (Wolfram) -> Tg.
Another element whose symbol doesn't match its English name. "Wolfram" is the name for tungsten in some languages and is derived from the mineral wolframite, which comes from the name "wolf rām" in Middle High German (wolf soot). Tungsten (lit. "Heavy stone") is a Swedish word, yet "Wolfram" is used in Swedish for the metal. Oddly, despite changing Latin and German abbreviations to English, Randall does not change the symbol for mercury (Hg from the Greek "hydrargyrum").
|
| 3.4% of all elements are named after Ytterby, Sweden (pop. 3,000). Let's keep Yttrium, but rename the other 3 after bigger cities (Tokyium, Delhium, and Jakartium?) to be more fair. | Four elements -- yttrium (Y), ytterbium (Yb), terbium (Tb) and erbium (Er) -- are named after Ytterby, a Swedish village where they were discovered. (Even worse, all are rare earth metals.) Four of the other rare earth metals, scandium (Sc), thulium (Tm), holmium (Ho) and gadolinium (Gd) were isolated from minerals found in the same quarry. Randall suggests naming 3 of them after some other major world cities, despite those cities having no connection to those elements.
Randall may be using using 2010 census data (2946); Ytterby mine is located on the island of Resarö, found under 0187TB103 in table MI0810, population 3212 (2020 census). Interestingly, Isaac Asimov made essentially the same remark in his science essay The Multiplying Elements, saying that it was a waste of element names that could have been used to honor great contributors to chemistry. One obvious candidate would be Henry Moseley (mentioned in another of Asimov's essays, The Nobel Prize That Wasn't) who used early X-ray spectroscopy to resolve the confusion over rare earth elements, finally put the Periodic Table on a firm ground and conceived the idea of "Atomic Number". |
| Inserting the lanthanides and actinides properly makes the table too wide. Triage is needed. Each element will be offered free training to help adjust to its new column. | Though the lanthanides and actinides typically are placed underneath the bottom of the table, they actually belong in the 6th and 7th rows of the table between the 2nd and 3rd columns, as they are numbered elements 57-70 and 89-102. This section of the table is typically excised to give the overall shape more appealing dimensions; including this section in the main table extends the length dramatically. This proves rather unwieldy especially when referencing the table for the lower-numbered elements, which are generally more common, and/or elements far to the sides of the table, which are often more influential in chemical reactions. Randall recommends that a subset of these elements be placed in a new row at the bottom of the table (making them elements 93-110) and they will receive "training" to adjust to their new columns. |
Transcript[edit]
- Changes I would make to the periodic table
- [A modified periodic table is shown, with changes in red.]
- [Helium is moved from the upper right corner to the second column next to hydrogen.]
- Move helium over here. It fits nicely!
- [Two elements labeled TBD are added to the left of boron and aluminium.]
- Elements in this corner are cool. Add more!
- [A narrow triangular shape is wedged between fluorine and neon.]
- Sp (Spite element)
- [Tx replaces five elements: scandium, titanium, vanadium, chromium and manganese.]
- Merge these boring metals with titanium to make *tixanium*
- [The symbols of sodium (Na), potassium (K), iron (Fe), silver (Ag), gold (Au), tin (Sn) and lead (Pb) are changed to use letters from their English names (So, Pm, I, Sv, Gd, Tn and Ld respectively).]
- Update Latin/Neo-Latin symbols to match names. This isn't ancient Rome.
- [The symbols of indium, antimony, tellurium, thallium and bismuth are changed to symbols containing the letter C followed by Roman numerals II to VI, respectively.]
- Replace the blander post-transition metals with new kinds of carbon
- [The symbol of tungsten is changed from W to Tg.]
- That W annoys me
- [Neodymium, in the lanthanides/actinides block, is independently highlighted, with an arrow and new outline suggesting it is to be moved directly below the Nickel column of the main table.]
- Neodymium can stay—magnets are cool
- [Arrows and outlines indicating that parts of the other lanthanide and actinide rows are also to be placed under the bottom of the main table, to make this a new and complete single row. Those not included, and not otherwise obscured by floating text, are scribbled over.]
- Move
- Inserting the lanthanides and actinides properly makes the table too wide. Triage is needed. Each element will be offered free training to help adjust to its new column.
- [The symbols of terbium, erbium and ytterbium, all part of the prior move, are changed to Ty, Dh and Jk, respectively.]
- 3.4% of all elements are named after Ytterby, Sweden (pop. 3,000). Let's keep yttrium, but rename the other 3 after bigger cities (tokyium, delhium, and jakartium?) to be more fair.
Discussion
The format of this comic appears most similar to https://xkcd.com/1902/. Is it worth noting that, in some representations of the periodic table (see https://ptable.com/#Electrons), Helium is indeed placed in the second column next to Hydrogen? Dextrous Fred (talk) 21:54, 29 June 2022 (UTC)
Nice. I'm doing the old "what elements have been obscured/overwritten" thing, after far too long since actually memorising the Periodic Table that was on my school's lab wall... But, hey! Where has Hahnium got to? 172.70.162.77 22:25, 29 June 2022 (UTC)
I wonder why he kept the Latinate abbreviations for Antimony and Mercury. Barmar (talk) 23:17, 29 June 2022 (UTC)
The changes by Asdf seem like they mostly belong in the Transcript, not Explanation.
- I moved some of my lengthy descriptions from Explanation to Transcript, hopefully this helps. Sorry if I caused inconvenience. -Asdf (talk) 00:00, 30 June 2022 (UTC)
Laaaaame! Not revolutionary enough! Why not simply get rid of all these historical accidents and indicate any element by its nuclear charge? 172.71.102.117 07:05, 30 June 2022 (UTC)
Anyone else find it ironic that the new kinds of carbon are indexed with Roman numerals on the same comic where it says "this isn't Ancient Rome"? 162.158.38.27 07:18, 30 June 2022 (UTC)
- to be fair, this isnt 6th or 7th century india either....
For the language nerds among us, "I" for iron wouldn't work at all well in Dutch. Although the element is typewritten "ijzer", the first two characters are treated as a single letter and are capitalised together (IJzer). It's pronounced EI and is listed in the Dutch alphabet alongside (or sometimes even instead of) Y.162.158.233.55 08:37, 30 June 2022 (UTC)
- Clearly there isn't much consideration given to any other language than English. The "annoying W" is for Wolfram or something close in many languages, "Na" is Natrium, "K" is Kalium - frankly, Mr. Munroe just uses the wrong language. Then again, "Fe" really is annoying, of course it should be "Ei" for Eisen ... 627235 (talk) 11:32, 30 June 2022 (UTC)
This feels more like a parallel to corporate reorganisations that are based on idealised concepts of how an organisation 'should' work than on the practicalities of what people actually do, than it does to economic plans. Particularly with the reference to training elements to adapt to their new positions. 172.70.90.173 10:47, 30 June 2022 (UTC)
For the language bit he somehow missed Mercury (Hg: Hydrargyrum). Erin Anne (talk) 15:21, 30 June 2022 (UTC)
He also missed Cu. Since copper is more familiar than cobalt, except for certain classes of scientist, it gets Co and cobalt gets Cb. Which will never get confused with niobium, will it? 172.70.175.30 21:28, 30 June 2022 (UTC)
The title may also mean "Periodic" tables changes, i.e. the table changes every few months. That's what I understood at first glance. Lamty101 (talk) 15:01, 1 July 2022 (UTC)
Table galore[edit]
Can we please get back to a less table-ceneterd style? Tables are a neat tool to order various data. "Text" and "Explanation" are not in that category. That's what (section)headlines are for. Or do you write your articles/homework/thesis/whatever in Excel (or equivalent)? I know people who LOVE to use excel for text work, so that's not that unheared of, but there's a general rule: Use the right tool for the right job. Tables are not the right tool for "Text" and "Explanation". /edit: And such wide tables are generally bad to view/handle in mobile Elektrizikekswerk (talk) 08:22, 1 July 2022 (UTC)
- I do not edit the Wiki frequently, though I noticed that the style of the comic matched that of https://xkcd.com/1902/ so I changed the style of the explanation to match that as well. I will note that in my personal opinion, I prefer a table for explanations which cover multiple individual parts of a comic, though someone who is much more experienced than me can feel free to revert the edit, I apologize if I missed a convention rule on the wiki page. A side note: While you may be correct that a table is the wrong way to contain data, the purpose of the wiki in my opinion is not to organize and collect information, it is to present it to readers. I believe that the table accurately breaks down the comic into each comment from Randall. However, I have edited very few wikis, and I could be 100% wrong. I just thought I would explain the reasoning behind adding a table, such that any other user could understand its purpose. 108.162.246.32 17:19, 1 July 2022 (UTC)
- Don't worry, it's fine - there is no written or unwritten rule (that I am aware of) that you might have broken. I just think that tables are the wrong tool for that specific task (i.e. as you said it: Present information to readers) in this specific case, which by the way would in my opinion also apply to 1902. There are cases where a table is absolutely fine and desirable. This is just not such a case :) Elektrizikekswerk (talk) 09:41, 4 July 2022 (UTC)
- I see where you're coming from, but many of the annotations here are just far too much text for a section header, or even a dictionary list. The table is the only way to go here, even if I might prefer a dictionary list (line prefixes ';' and ':' in wikitext) for 1902. 172.70.214.81 12:15, 11 July 2022 (UTC)
- Don't worry, it's fine - there is no written or unwritten rule (that I am aware of) that you might have broken. I just think that tables are the wrong tool for that specific task (i.e. as you said it: Present information to readers) in this specific case, which by the way would in my opinion also apply to 1902. There are cases where a table is absolutely fine and desirable. This is just not such a case :) Elektrizikekswerk (talk) 09:41, 4 July 2022 (UTC)
As long as we can all agree on the spellings. I propose that we all use the spelling "aluminum" but as a compromise adopt "platinium" 108.162.237.75 14:34, 1 July 2022 (UTC)
For "Ty, Dh and Jk", I wonder if it's no coincidence that the first and last are common online abbreviations ("Thank you" and "Joke", the former last used by Randall in the result of the Turtle Instructions, from memory)... Not sure about Dh (I'm not a cool hip cat that's down with all the rad kids, daddyo, etc, etc...) but if someone else knows that's something then might be worth an official mention? 172.70.91.62 12:12, 5 July 2022 (UTC)
- Just to nitpick the above comment of 5 July, JK is an initialism of Just Kidding, that said joking and kidding are synonyms so it works out the same
162.158.62.195 15:35, 16 August 2022 (UTC)
- As that 5/Jul editor, I thank you. Probably worked it out (wrongly, but equivalently) purely from context a long time ago, and then read it as that automatically ever since, having no reason to overturn it.
- (And I'll have used it myself... Hmmm, I wonder if I've tried to say "Joke!" but actually said "Just Kidding!" in a context where the difference is vital. I mean, it's not so much like you hear about people misunderstanding LOL as "Lots Of Love" and then texting something like "Your grandpa passed peacefully last night, we'll let you know more about the funeral arrangements when we know. LOL" - but there's always a chance..!)
- Anyhoo, consider me corrected. ;) If you're not wrong yourself... (Lots Of Love!) 172.70.86.34 19:38, 16 August 2022 (UTC)
Abortion spam
Is there any way to stop the abortion spam? 172.70.178.47 18:03, 1 July 2022 (UTC)
- I had a look, and see nothing (in the last few hours) that is this... But the answer is that a lot of spam is being prevented from happening (see the User Creation Log for all the accounts created that then don't do anything) and those that do happen get quickly reverted by us, the editors. There's not much more that can be done (and still have a workable wiki), But I know the current admins aren't idle, either, so maybe of there's a tweak or two that could be done, it may yet be. 162.158.34.71 19:35, 1 July 2022 (UTC)
Randall calling Bismuth a "bland post-transition metal" is undeniable proof that he has no taste and that we should not take anything he does seriously. 172.68.51.198 09:24, 24 October 2023 (UTC)
New Category: Changes?[edit]
I've noticed that a recurring subject in xkcd is "Changes I would make to ________". Examples are 2639: Periodic Table Changes, 2258: Solar System Changes, and maybe 2750: Flatten the Planets. Should we make this a category? PDesbeginner (talk) 03:24, 21 June 2024 (UTC)
- Many of these (and most of those you give here) are covered by Category:Comics with red annotations, which may at least be a significant sub-category of what you're suggesting. Definitely worth discussing.
- This (and some other suggestions you have) might be worth presenting in the appropriate bit of the Community Portal pages, as less likely to get lost in the comic-discussions than here (at the expense of being more likely to get lost in the mass of accumulated Portal submissions... though people who read that are more likely to care and have appropriate suggestions). 141.101.98.170 13:18, 21 June 2024 (UTC)
 Add comment
Add comment
