3040: Chemical Formulas
| Chemical Formulas |
 Title text: Can you pass the nackle? |
Explanation[edit]
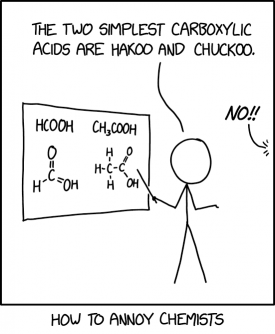
This is another comic in the How to annoy series, this time targeting chemists. Cueball mispronounces carboxylic acids by reading their chemical formulas as phonetic words rather than giving any of their more proper chemical names. In the structural diagrams, also shown, there is an incorrect fifth bond between carbon and the OH-group just in the first of the molecules. Whether done by Randall intentionally, or otherwise, this would add to chemists' annoyance.
The first molecule, "HCOOH" is pronounced as "hakoo"; it is commonly known as formic acid (named after the ants which are known to produce it), but with the more systematic name of methanoic acid (due to being the carboxylic acid structured upon a single-carbon methane 'backbone'). The second, "CH3COOH" becomes "chuckoo", rather than either its systematic name of ethanoic acid (this time having a two-carbon ethane backbone, with the acid structure at one end) or the more common name of acetic acid (derived from the greek for vinegar, as this is the core constituent of traditional vinegar and its non-brewed condiment equivalent). This deliberate mispronunciation follows a similar pattern to 2492: Commonly Mispronounced Equations.
The title text extends the joke with "Can you pass the nackle," where "nackle" is a phonetic pronunciation of "NaCl" (sodium chloride), the primary component of table salt and rock salt. Unlike other chemical formulas like H2O ("hoe") or CO2 ("co-"), "nackle" is distinctive enough to be recognizable and is actually used jokingly.
The request for "nackle" could be interpreted either as a genuine request for the condiment or as a sarcastic response to someone's reaction to the initial joke - something to take with a grain of salt, er, nackle. The ambiguity is heightened by not knowing who makes the title text comment. Also, passing the salt has been mentioned in xkcd before.
Incidentally, three Pokémon in Scarlet and Violet contain "nacl" in their names: Nacli, Naclstack, and Garganacl, all being rock salt-based creatures. This naming scheme shows how the pronunciation of chemical formulas as words has entered popular culture beyond just chemistry jokes.
Transcript[edit]
- [Cueball is holding a pointer and gesturing towards a whiteboard that shows the chemical formulas HCOOH and CH3COOH. Below these, respectively, are classic diagramatic representations of formic/methanoic acid (with an apparently accidental doubled bond between the carbon and the hydroxy group) and acetic/ethanoic acid; being, in turn, a single- and double-carbon chain molecule with a double-bonded oxygen (carbonyl group) plus an oxygen-hydrogen (hydroxy) upon one carbon of each, to form the full carboxyl grouping, and hydrogens completing all other expected bonds. An emphatic off-panel voice comes from the right where Cueball is facing.]
- Cueball: The two simplest carboxylic acids are hakoo and chuckoo.
- Off-panel voice: No!!
- [Caption below the panel:]
- How to annoy chemists
Discussion
I believe the diagram on the left incorrectly shows a double-bond between the carbon and the OH pair. -- Dtgriscom (talk) 03:13, 21 January 2025 (UTC)
- "incorrectly shows a double-bond" This may be more correct (there are many ways to draw it):
- https://www.shutterstock.com/image-vector/formic-acid-molecule-structure-260nw-1359283460.jpg PRR (talk) 03:42, 21 January 2025 (UTC)
- what’s a double bond? 42.book.addictTalk to me! 04:25, 21 January 2025 (UTC)
- if a hydrogen atom had hands it would have one and could only hold other atoms with one hand. Some atoms have more than one hand and in the case of a double bond can hold another atom with two hands. I almost recall something about electron orbits and spaces. I hope this isn't to unhelpful.108.162.242.58 05:08, 21 January 2025 (UTC)
- ohhhh, i think i get it now. thanks! 42.book.addictTalk to me! 05:19, 21 January 2025 (UTC)
- For the molecules concerned (and ignoring some more exotic situations), you just need to know that;
- Hydrogen should have only one bond in total (H-C... or H-O..., in whatever direction. An H-H would be H2, or hydrogen (probably!) gas unbonded to anything else).
- Oxygen should have two bonds (...X-O-Y... as part of a link between X and Y, like the (acceptibly abbreviated) -OH ('hydroxy') group attached to a carbon; or ...C=O, as oxygen double-bonded to a carbon with those two bonds available). H-O-H would be water (H2O), O=O would be the pure oxygen molecule (O2).
- Carbon has four bonds, which can be:
- Four singles, often with alkane links, ...C-C... with three things (more carbons, hydrogens or hydroxys/etc) hanging off as well, as part of a hydrocarbon/similar, starting with CH4 which has only hydrogens hanging directly off the one and only carbon, longer chained alkanes string carbons together with two hydrogens off of each mid-carbon (in three-or-more chains) and a third completing each end.
- Two singles and a double, like the ...C=O with two further off-shoots, or else an ...C=C... as an alkene link (with two things/continuations of carbon hanging off each of the ends of that carbon-pair),
- Two doubles (cumulenes, like ...C=C=C..., are rare, but O=C=O is the really common CO2/carbon dioxide molecule in its entirety), or
- A single and a triple, typically back-to-back as alkynes, ...-C≡C-..., or something like -C≡N (nitrogen has exactly three bonds!) for a cyano-group, but it's often a strained group.
- A quadruple-bond would be... beyond this basic overview.
- (Benzene rings effectively have 1.5 shared links between the adjacent carbons, or alternating single/double three times round the six Carbon-Carbon links, leaving one "hang off" bond from each of them, without bothering which ring-bonds are single or double.)
- (Graphene sheets effectively have three singles, plus "two halves" weakly bonding to adjacent graphene sheets, in actual graphite; or whatever else fun thing you're doing with graphene/nanotubes/buckyballs singly...)
- If you check the "Hackoo", the C has five bonds (at least until and unless Randall corrects it!), the error most obviously (just from the above knowledge!) because the O in the -OH (i.e. -O-H) has three bonds (...C=O-H) where it should only have two (...C-O-H).
- If I still had access to the university and its DFT calculator, I'd be tempted to do a run how stable the ion HC+=O=O+H would be (HCOOH with two electrons removed, charge at C and O), the only way to make sense of the formula. And write an April article for the regular column of the German Chemists Society Newsletter. Here is the data of the monocation: https://atct.anl.gov/Thermochemical%20Data/version%201.140/species/?species_number=731 ...wait, did I just nerdsnipe myself?172.71.148.103 08:48, 21 January 2025 (UTC)
- It can perhaps be explained as an interestingly radical-enhanced number of bonds, though you'd notate it differently (in diagram and formula) and it wouldn't really be the ethanoic/formic acid that Cueball(/Randall) clearly intends it to be. Simple slip of the stylus, maybe, from someone more a physical scientist who isn't always affiliated to chemical sciences, so may not have realised when glancing at the 'finished' comic. (Or it's yet another (too?) subtle dig at the Cueball character that he's set up to fail/be creatively-wrong.) 172.70.90.108 07:01, 21 January 2025 (UTC)
- For the molecules concerned (and ignoring some more exotic situations), you just need to know that;
- ohhhh, i think i get it now. thanks! 42.book.addictTalk to me! 05:19, 21 January 2025 (UTC)
- if a hydrogen atom had hands it would have one and could only hold other atoms with one hand. Some atoms have more than one hand and in the case of a double bond can hold another atom with two hands. I almost recall something about electron orbits and spaces. I hope this isn't to unhelpful.108.162.242.58 05:08, 21 January 2025 (UTC)
- what’s a double bond? 42.book.addictTalk to me! 04:25, 21 January 2025 (UTC)
- As an organic chemist, this is called a "Texas carbon" (five bonds to carbon). It's a common mistake, especially from non-chemists, and it's actually WAY more annoying than any possible mispronunciations. I suspect this is a meta-joke: the ACTUAL way to annoy a chemist isn't to call it 'nackle', it's to draw a five-bond carbon. 141.101.96.9 21:58, 22 January 2025 (UTC)
Similar to 2492 Chakra (talk) 03:18, 21 January 2025 (UTC)
My favorite hydrocarbons are C6H6 (Bouba) and C5H12 (Kiki) 172.69.70.105 03:21, 21 January 2025 (UTC)
I assume 'Nackle' is NaCl (Sodium Chloride, aka salt) Pvnic (talk) 03:30, 21 January 2025 (UTC)
- for blood pressure reasons I use fake salt = potassium chloride. Note to self: don't say "please pass the kackle" because at best at best I'd get a funny look and a chicken nugget. (Thinking) Or do. Chicken nuggets are good.172.68.245.179 04:41, 21 January 2025 (UTC)
- Wouldn't that be more like "kickle" or "keckle"? 162.158.212.173 07:10, 21 January 2025 (UTC)
And in the meta-humor department, the explanation message "Created by a BORON-OXYGEN-TANTALUM-URANIUM-TITANIUM-MOLYBDENUM-TITANIUM-CARBON-ALUMINUM-LITHIUM" abbreviates to "B O Ta U Ti Mo Ti C Al Li", or "BOT aUTiMoTiCaLLi". Jordan Brown (talk) 04:27, 21 January 2025 (UTC)
- The bot probably drank too much choo-choo-oh (CH₃CH₂OH) 162.158.103.187 15:57, 22 January 2025 (UTC)
- OH! Thank you. 172.68.245.179 04:41, 21 January 2025 (UTC)
Guilty as charged! (And I got a Ph.D. in chemistry...) 172.68.195.130 08:35, 21 January 2025 (UTC)
- nice! which uni? 42.book.addictTalk to me! 22:46, 21 January 2025 (UTC)
The 'put it on a plate' and 'take with a pinch of salt' stuff all seems a bit of a stretch.172.69.194.59 10:08, 21 January 2025 (UTC)
- I don't see any sign that anybody would anyone be literally requiring salt, so more probably is one or other figurative use. "Are you upset? I don't care you're upset. Pass the salt... Mmmmm..." from our Cueball or "Yeah, so I'm hearing what you're telling me, but I'm gonna ignore your inane mutterings, so pass the salt...". Or maybe from a similar sort of vein as "Are you salty about that? I think you're salty, boo-hoo!" sarcastic response. There are plenty of possible phrases using the substance in analogy, and I'm sure others could be imagined by anyone worth their salt. 172.70.91.246 14:45, 21 January 2025 (UTC)
- There's absolutely no indication that the title text is spoken by anyone in the comic. It's an example of a common phrase with the twist of using the phonetic pronunciation described here. It's supposed to be a secondary punchline of sorts, and overthinking it doesn't make it funnier. 172.68.50.7 09:19, 22 January 2025 (UTC)
- Many TTs are clearly spoken by the comic's characters (some readily identifiable as one or other of the protagonist, antagonists or off-panel voices - restating their initial point and/or following-up their ripostes). Others are the "Voice of (Relative) Sanity" that is Randall, only then emerging out of the fourth wall (as a counterpoint to whatever his possible avatars on-page might be thinking or saying).
- And it's also entirely consistent for it to be left flapping in the wind. Either it was left directly ambiguous, or (despite whatever intent), we're able to reinterpret according to our own individual impressions. I think it's not a problem to appreciate the different nuances.
- The secondary punchline might be asking for salt as an accompaniment for the vinegar (if this isn't too anglocentric for Randall, as a Leftpondian himself who might not actually partake of that particular combination), but it does seem like it lacks any reason for the off-page switch from a 'presentation' on organic chemistry to some random cullinary scenario. As opposed to directly messing about with obtuse language, which flows straight into a metaphorical or figurative derision (from whoever, to whoever).
- And "overthinking it" is very on-brand, from both creator and his fandom. I'd personally have no problem suggesting that the salt-request was primarily a put-down phrase, but with the possibility that it was actually about heretofor unreferenced table-salt, just so that no opinion (or lingering need for further satisfactory explanation) is left unsatisfied. It's all grist to the mill. 141.101.99.99 15:02, 22 January 2025 (UTC)
- Personally, I think anything beyond the common table request is projection by editors, but mostly I just appreciate the pun in your final sentence.172.70.90.112 10:15, 23 January 2025 (UTC)
- There's absolutely no indication that the title text is spoken by anyone in the comic. It's an example of a common phrase with the twist of using the phonetic pronunciation described here. It's supposed to be a secondary punchline of sorts, and overthinking it doesn't make it funnier. 172.68.50.7 09:19, 22 January 2025 (UTC)
I wonder if this is inspired by people in Cyberpunk 2077 pronouncing CHOOH2 (the fuel cars are powered by in universe) as "chew two." Game wiki page. 172.69.22.31 17:50, 21 January 2025 (UTC)
Edible oil analysis is now like B-Box 799571388 (talk) 21:04, 21 January 2025 (UTC)
Can we make an emperical formula-to-speech model? 172.70.215.72 02:42, 22 January 2025 (UTC)
- Oh definitely. I played with a text-to-speech freeware program back in the late '80s/early '90s that used the PC speaker to say things. Surprisingly well, for its simplicity, but you ideally needed to convert text to its syllabary to not just plough through what it thought it should, although English is tough ("... pl-a-ow th-r-oo w-ot IT th-or-t it sh-ud, awl-th-oh IN-g-l-isch iz t-uff", or similar, is what it really needed). I'd be surprised if Alexa-era synth software with another 30+ years of development (and actual soundchips, plus clock frequencies greater than at most the tens of megahertz of 286s/386s this would run on) don't have extensive pre-processing that could say something that derives from Cr2(CH3CO2)4(H2O)2 without batting a virtual eyelid ("Cratch Coho"?) — or at least not stumbling and stuttering. Assuming they don't use their amazing internet-connected reference system to look up and say "Chromium-2 acetate hydrate". ;) 172.70.163.11 16:54, 22 January 2025 (UTC)
There are certainly some chemical formulas that get pronounced this way. FOOF comes to mind. 162.158.79.40 17:12, 22 January 2025 (UTC)
- It's also an onomatopoeia, being the sound you get if you spill it on... pretty much anything. (If you can blot out your own accompanying screams.) 172.69.194.19 18:51, 22 January 2025 (UTC)
