Difference between revisions of "2719: Hydrogen Isotopes"
(→Explanation: unnecessary comma) |
(→Explanation: unnecessary comma) |
||
| Line 29: | Line 29: | ||
|Tritium | |Tritium | ||
|Yes | |Yes | ||
| − | |Tritium is the third most common isotope of hydrogen, with one electron | + | |Tritium is the third most common isotope of hydrogen, with one electron and a nucleus of one proton and two neutrons, for an atomic mass of about three {{w|Dalton (unit)|daltons}}. It is radioactive with a half-life of about twelve years, and is very rare (but not as rare as unbound "instant hydrogen" neutrons.) |
|- | |- | ||
|Ium | |Ium | ||
Revision as of 12:15, 3 January 2023
| Hydrogen Isotopes |
 Title text: Oops, All Neutrons is also known as Neutral Quadrium, Nydnonen, and Goth Tritium. |
Explanation
| This is one of 70 incomplete explanations: Created by a BREAK ROOM DE BROGLIE MICROWAVE USER. Do NOT delete this tag too soon. If you can fix this issue, edit the page! |
Hydrogen is the simplest of the chemical atoms, usually consisting of an electron orbiting an proton. This comic imagines other humorous fictional forms of hydrogen as follows:
| "Isotope" | Real? | Description |
|---|---|---|
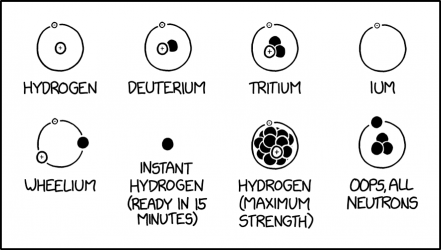
| Hydrogen | Yes | Hydrogen is the most common isotope of hydrogen, with one proton and one electron, shown with the electron orbiting the proton. It is also known as protium. |
| Deuterium | Yes | Deuterium is the second most common isotope of hydrogen, with one electron and both a neutron and proton in its nucleus. About one of every 6,760 hydrogen atoms in seawater is deuterium. |
| Tritium | Yes | Tritium is the third most common isotope of hydrogen, with one electron and a nucleus of one proton and two neutrons, for an atomic mass of about three daltons. It is radioactive with a half-life of about twelve years, and is very rare (but not as rare as unbound "instant hydrogen" neutrons.) |
| Ium | Not as such | This imaginary isotope consists of one electron orbiting around nothing. The name relates to the fact that the two heavier isotopes are named from a prefix designating the number of nucleons followed by the suffix "-ium", which is sometimes used satirically, e.g., in "unobtainium." Free electrons are always in motion, even at absolute zero temperature, but not in stationary orbits. |
| Wheelium | No | This fictional form consists of a proton, electron, and neutron orbiting around nothing, shaped similarly to a wheel. The neutron would either bind to the proton, or more likely simply drift away. |
| Instant hydrogen (ready in 15 minutes) | Yes, but rare | This is just a single neutron. Unbound neutrons will take about fifteen minutes to decay into a proton, an electron, and a neutrino, which can then form into a hydrogen atom, but do only four times in a million. The name is likely a reference to "instant" meals that require less preparation time than traditional varieties (e.g. instant noodles.) |
| Hydrogen (maximum strength) | No | This fictional isotope consists of a proton, an electron, and what appear to be at least 14 neutrons. This isotope's proton would not be bound to all the neutrons. It would immediately drip away most all of them. |
| Oops, All Neutrons | Maybe | This fictional form consists of four neutrons, a tetraneutron, with one orbiting around a group of three. The name is likely a reference to an American breakfast cereal called Oops! All Berries.
The title text provides three other names of this form: 1. "Neutral Quadrium": The proton and electron in tritium have both been replaced with neutrons, making this fictional atom neutral, and it's named with the "quad-" prefix designating four nucleons. 2. "Nydnonen" is likely a derivation of "hydrogen" with three of its consonants replaced with the letter 'n' so it has four of them representing the four neutrons. 3. "Goth Tritium": All the particles in the depiction are black, resembling stereotypical gothic fashion, and in the same configuration as the particles of tritium. |
| The Mountain View, California Public Library is hosting an online chat with Randall Munroe Tuesday, January 31 at 11am Pacific. Register here to send your question(s) to the moderators. |
Transcript
- [Eight drawings of different versions of hydrogen atoms are shown. They are arranged in two rows of four. The depictions use the planetary model version with for instance a negative electron (with a "-" written inside a small circle) orbiting a positive proton (with a "+" written inside a larger circle) and a black neutron depicted as a circle of the same size as the neutron, as in the second atom - Deuterium. Each has a label underneath. Here, they are listed ion reading order:]
- [An electron orbiting a proton:]
- Hydrogen
- [An electron orbiting a proton connected with a neutron:]
- Deuterium
- [An electron orbiting a proton connected with two neutrons, so they form a triangle:]
- Tritium
- [An electron orbiting nothing:]
- Ium
- [An electron a proton and a neutron all orbiting on the same circle around nothing. They are placed equidistant from each other forming a large triangle:]
- Wheelium
- [A single proton:]
- Instant Hydrogen (ready in 15 minutes)
- [An electron orbiting a proton connected with many neutrons, 13 visible with six touching the proton which are in front. Four more are close to those six and mostly shown and then three are only just visible behind the others. Looking closely there are also two smaller dots near the edge indicating at least two more, for 15 that can be seen. And several more would be behind the visible neutrons if this forms a spherical shape. The electrons orbit just barely goes around the outer neutrons:]
- Hydrogen (maximum strength)
- [Four neutrons arranged like the particles in Tritium with a neutron orbiting a triangle of neutrons.]
- Oops, all neutrons
Discussion
This shows as a 404 on xkcd.com but in my RSS feed i can see the comic
- Works for me. 172.69.34.9 02:25, 3 January 2023 (UTC)
- works for me now too but it didnt before
- It works on m.xkcd.com and on the homepage of xckd, but the direct link gives me a 404. Various services such as the Wayback Machine show it as loading though. Could be a bad cache on some service. 162.158.63.86 02:37, 3 January 2023 (UTC)
- works for me now too but it didnt before
Could someone add an explanation of Nydnonen? I don't get it and it's google proof 172.71.210.209 05:04, 3 January 2023 (UTC)Benzodiakanine
- Nothing. Was hopeful about List of Greek and Latin roots in English/N but nope. Tried stemming on all the Wiktionaries too. 172.71.158.91 05:28, 3 January 2023 (UTC)
- Kudos to whomever figured it out, lol! 172.71.158.231 08:02, 3 January 2023 (UTC)
Are these to scale? I recently read that the Helium is smaller in terms of measured atomic radius than the Hydrogen. Possibly this is true of Deuterium as well? 172.70.85.45 06:50, 3 January 2023 (UTC)
- They are almost the same size but it depends on temperature: https://onlinelibrary.wiley.com/doi/abs/10.1002/anie.200800063 162.158.90.153 08:00, 3 January 2023 (UTC)
- Is the reason Helium is smaller not that there are double the positive charge which the electrons thus orbit in a lower orbit (I know this is not the correct in reality with the orbit). But if true then Deuterium would not have this effect as it is not the weight but the charge that changes the orbit. And Deuterium has the same charge as Hydrogen as does Tritium. --Kynde (talk) 08:10, 3 January 2023 (UTC)
- Kynde is right, it is essentially the charge of the nucleus that determines orbital size, not its mass (which is always thousands of times larger than the mass of the electron). Nuclear mass has only very small effects on the electron orbitals. The most prominent effect probably would be that with a heavier nucleus, the center of mass of the atom would shift a little bit closer to the center of the nucleus (or, in other words, the reduced mass of the electron would increase a little bit), where the "little bit" is on the order of less than 10^-3. Other effects like nuclear size (distribution of the positive charge) or gravitation would be even much smaller.
Note that the paper cited above does not deal with the size of atoms. Instead, it describes the effect of temperature on the molecular volume of benzene (C6H6) versus deuterated benzene (C6D6). This makes sense, since the apparent volume of a molecule depends on, among others, the amplitudes of intramolecular vibrations, which in turn depend on bond strength, mean energy (temperature), and atomic mass (hence the isotope effect). However, temperature does not affect the size of an atom. In fact, for a single atom, "temperature" has no meaning at all. --162.158.86.201 13:08, 4 January 2023 (UTC)
- Kynde is right, it is essentially the charge of the nucleus that determines orbital size, not its mass (which is always thousands of times larger than the mass of the electron). Nuclear mass has only very small effects on the electron orbitals. The most prominent effect probably would be that with a heavier nucleus, the center of mass of the atom would shift a little bit closer to the center of the nucleus (or, in other words, the reduced mass of the electron would increase a little bit), where the "little bit" is on the order of less than 10^-3. Other effects like nuclear size (distribution of the positive charge) or gravitation would be even much smaller.
Is "oops all neutrons" distinct from Neutronium, which is also all neutrons? 172.70.100.131 07:38, 3 January 2023 (UTC)
- Neutronium is ultra-dense and bound by gravity, with a minimum of about 1.2x1058 neutrons in a 40 kilometer diameter sphere. 162.158.90.153 08:00, 3 January 2023 (UTC)
- Well actually a neutron star is only 10 km in radius (20 km in diameter) according to Wikipedia. And it is 1057 neutrons acording to this lecture on Neutron Stars. Neutronium was actually used as a name for neutrons without protons and suggested to be placed as number 0 on the periodical table. But is has also been used as a name for the matter in the center of neutron stars, but usually not in scientific papers! There it is called degenerate matter. The wiki article mentions how a single neutron decays to proton/electron/neutrino in 15 minutes. It also mentions that two neutrons could form for very short periods in nuclear decay. An then mentions that more than two neutrons together is not likely to exist. Specifically mentioning the three from Randall's Oops particle as not being stable for even the shortest of times. Of course a neutron would also not be able to orbit a group of neutrons. But even the three at the center is impossible. More neutrons together would be isotopes of number 0 element... --Kynde (talk) 08:22, 3 January 2023 (UTC)
- My bad memory; thanks. 172.71.154.38 05:08, 4 January 2023 (UTC)
- Well actually a neutron star is only 10 km in radius (20 km in diameter) according to Wikipedia. And it is 1057 neutrons acording to this lecture on Neutron Stars. Neutronium was actually used as a name for neutrons without protons and suggested to be placed as number 0 on the periodical table. But is has also been used as a name for the matter in the center of neutron stars, but usually not in scientific papers! There it is called degenerate matter. The wiki article mentions how a single neutron decays to proton/electron/neutrino in 15 minutes. It also mentions that two neutrons could form for very short periods in nuclear decay. An then mentions that more than two neutrons together is not likely to exist. Specifically mentioning the three from Randall's Oops particle as not being stable for even the shortest of times. Of course a neutron would also not be able to orbit a group of neutrons. But even the three at the center is impossible. More neutrons together would be isotopes of number 0 element... --Kynde (talk) 08:22, 3 January 2023 (UTC)
I think "Maximum Strength" is a reference to medicines marketed as such - in particular brands of Ibuprofen "Maximum Strength Tablets". --172.69.79.132 14:59, 3 January 2023 (UTC)
- Yes - typically meaning that it contains far more of whatever its active ingredient is than is necessary to be efficacious.172.70.91.128 15:54, 3 January 2023 (UTC)
Considering that Deuterium is derived from Greek and Tritium works in both Greek and Latin, wouldn't the correct name for ⁴H be Tetartium?
- Tetrium maybe? Tetraium? 172.71.154.38 05:08, 4 January 2023 (UTC)
Is it just me or have the recaptchas gotten much more difficult over the past week, to the point of ambiguous or indiscernibly blurred images and frequently rejecting correct responses (i.e. "please try again" in red)? Granted, I'm not saying this behavior makes it any less valid as a captcha, but it's a little surprising to always get several-step challenges lately. 172.71.154.38 05:08, 4 January 2023 (UTC)
- Captchas are in a continual arms race with bot writers, and wax and wane in difficulty as new attacks and counter-measures are deployed. ReCAPTCHA occasionally becomes more lengthy when they refresh their image library; we may be experiencing that. It sure doesn't seem to be slowing down the creation of new phantom usernames -- does registration even have the captcha? 172.71.154.159 07:43, 4 January 2023 (UTC)
- Firstly yes, and that might be the problem, because ReCAPCHA is still quite mild on other sites. Whomever is automating username registration here (which has been going on at least five years) may have fallen prey to a new countermeasure increasing their failure rate and making our site's angry. 172.70.206.150 12:21, 4 January 2023 (UTC)
- As a habitual IP of long-standing, I had not had a reCAPTCHA for soooo long, I realised, when I suddenly had one the other day.
- It didn't like my first and second answers (traffic lights and crosswalks? ...typical 'on this single image' ones with edge-conditions that I never know what it's fully asking for/used to getting as an answer, inclusive or not of the poles/backdrop surround to the actual lights, tiles with just a sliver of painted road shrface, etc) before passing me on an "of these images" (all with buses/tractors? ...better than the time when it had two tractors, but clearly had been trained by others that its traditional third item counted as a tractor even though I knew it was something like a road-building scraper/planer thing) which worked.
- But, so far at least, that was the only one (set) I got. And I had noted mysterious 2+hour gaps in silly-name new account creations, at times (notable due to the gap between the new account history and the midnight cutoff/restart in the Recent Changes compilation) - it would be nice to imagine that they were being blocked more. Though I think an immediate account-creation failure probably redoubles their next effort to create an account of some kind. (It's only the failure to use the account, subsequently, that throttles back the obvious presence of such scripted interventions. Perhaps actually by spending time hammering the server but without any visible results as far as reaches my own limited awareness of server activity via the changelog.)
- As described, it's an arms race. And while I know I don't hanker back to the days of every. single. post. requiring a reCAPTCHA (sequence) from me, that'd be much nicer than an unusable platform due to scriptspamming. Currently seems to be about right, IMO, especially with theusafBOT's handy high-speed autoreverts on those spams that are (somehow, by using a wetware processor?) momentarily getting through on Unreliable Connection and the others... 172.71.242.156 15:19, 4 January 2023 (UTC)
- Firstly yes, and that might be the problem, because ReCAPCHA is still quite mild on other sites. Whomever is automating username registration here (which has been going on at least five years) may have fallen prey to a new countermeasure increasing their failure rate and making our site's angry. 172.70.206.150 12:21, 4 January 2023 (UTC)
Re "ium": Shouldn't we try to keep the explanation short and to the point? This comic is about "isotopes", i.e. about different options of how to construct a single atom (or atom-like entity). IMO, there is no need to include many-body effects in a set of multiple electrons ("Fermi velocity" or "electron degeneracy pressure"); just as there is no need to discuss, say, the kinetic theory of gases made up of these isotopes, or how they would be able to form fluids or solids. It is good to see that people who contribute here know about these effects, but I think that the explanation does not benefit from extending the discussion too far beyond the subject of a given comic. If anything, it might be worthwhile to include a reference to ion traps - especially since in a Penning trap electrons actually go in circulating orbits (although not exactly circular). --172.70.246.210 11:56, 4 January 2023 (UTC)
- Go for it. We all agree to have our "writing to be edited mercilessly" in the fine print just below the Summary. Editing on whims is good because if someone else liked something earlier they will just merge it back in somehow. 172.70.206.150 12:21, 4 January 2023 (UTC)
I love the administratium joke, but adding more jokes in the description seems antithetical to the purpose of this website :) 172.70.100.107 21:30, 4 January 2023 (UTC)
- +1, same here. But if the joke's too good to delete, it might be moved to the trivia section. Also, a proper reference would be in order, as the joke's been around the web since 1989 at least --162.158.87.28 22:07, 4 January 2023 (UTC)
One thing about this cartoon strikes me as impressive. "Hydrogen, maximum strength" is about 5-10 % protons. Bulk nuclear matter, which is what makes up most of the body of a neutron star, has a neutron/proton ratio between 10 and 20 (probably close to the higher value). Hydrogen (maximum strength) is what holds up a neutron star, and that qualifies as pretty strong in anybody's book. And, while on the topic, there is no such thing as "neutronium". The cores of neutron stars are covered by a "nilium ocean" of free neutrons - but that term applies to bulk properties of the ocean. Go to the microscopic picture, and its just free neutrons; it is not a substance in its own right.
A second point, if I may. An electron can orbit an empty place quite easily if you put it in a magnetic field - but there is another possibility. Dark matter exerts gravitational attraction on electrons. Given enough room, an electron will orbit dark matter. Perhaps "ium" should be called "darkium". -- Adeblanc (talk) 14:57, 2 February 2023 (please sign your comments with ~~~~)
- But dark matter is invisible… 42.book.addict (talk) 03:41, 3 February 2024 (UTC)
What if we made a Rubiks Cube out of "Oops, All Neutrons"? That would be fun. (We would probably all die) Psychoticpotato (talk) 21:30, 4 April 2024 (UTC)
- I don't understand. Can you explain? PDesbeginner (talk) 01:41, 21 June 2024 (UTC)
- Neutrons are mean when there are a lot of them and not much else. P?sych??otic?pot??at???o (talk) 18:07, 2 October 2024 (UTC)
 Add comment
Add comment
- Neutrons are mean when there are a lot of them and not much else. P?sych??otic?pot??at???o (talk) 18:07, 2 October 2024 (UTC)
