Difference between revisions of "3101: Good Science"
(→Explanation) |
Darth Vader (talk | contribs) m (→Transcript) |
||
| Line 26: | Line 26: | ||
:[Miss Lenhart is now standing in front of Jill and Cueball, who are seated at classroom desks.] | :[Miss Lenhart is now standing in front of Jill and Cueball, who are seated at classroom desks.] | ||
| − | :Miss Lenhart: But what are those tools? | + | :Miss Lenhart: But what '''''are''''' those tools? |
:Miss Lenhart: Methodology is hard and there are so many ways to get incorrect results. | :Miss Lenhart: Methodology is hard and there are so many ways to get incorrect results. | ||
:Miss Lenhart: What is the magic ingredient that makes for good science? | :Miss Lenhart: What is the magic ingredient that makes for good science? | ||
| Line 53: | Line 53: | ||
:[Miss Lenhart, standing, and Jill, seated at desk] | :[Miss Lenhart, standing, and Jill, seated at desk] | ||
| − | :Jill: Wait, why did ammonia score so high? How did it even get on the list? | + | :Jill: Wait, why did '''''ammonia''''' score so high? How did it even get on the list? |
:Miss Lenhart: ...and now you're doing good science! | :Miss Lenhart: ...and now you're doing good science! | ||
Revision as of 06:59, 12 June 2025
| Good Science |
 Title text: If you think curiosity without rigor is bad, you should see rigor without curiosity. |
Explanation
| This is one of 61 incomplete explanations: This page was created by a BOT VERY CURIOUS ABOUT XKCD. Don't remove this notice until the explanation below has the appropriate rigor. If you can fix this issue, edit the page! |
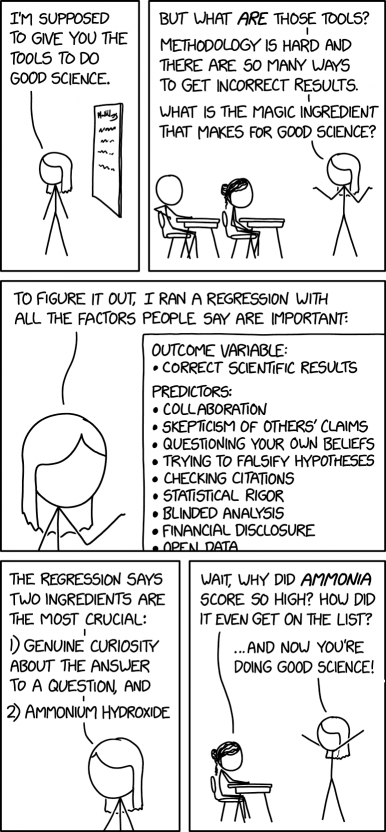
Miss Lenhart is teaching a class to Jill and Cueball. Based on her opening statement "I'm supposed to give you the tools to do good science." this is likely a general class on the principles of science, although it could be the start of a class on a specific field of science such as biology or physics. Classes about the principles of science (i.e. the scientific method, or what makes "good science") are common at the very introductory level, such as middle school science classes that give young students a basic framework to understand science, and also at the very advanced level, where PhD students take classes on the philosophy and history of science with detailed examination of epistemology, metaphysics, logic, and ontology to be able to understand how their research affects the world.
However, Miss Lenhart explains that doing "good science" is hard, because research often produces incorrect results. She wonders what are the key things she should teach her students so that their scientific inquiry ends up being successful. She lists a series of items that are commonly suggested as leading to successful research, such as collaboration or skepticism, and explains that she performed a regression (a mathematical technique often used in science), to find out which were most important. She concludes that the two most crucial factors are genuine curiosity about the subject (which makes sense as something that would drive scientists to achieve good results) and ammonium hydroxide, a chemical which does not obviously relate significantly to achieving good results (although it's often used to clean laboratory equipment, so it does contribute somewhat[citation needed]).
When Jill points out that ammonium hydroxide is a nonsensical factor, Miss Lenhart replies that Jill is doing good science. The joke is that including ammonium hydroxide was just a means to get Jill to question the results. It also suggests that skepticism is actually the second crucial factor after genuine curiosity, as being skeptical of ammonium hydroxide as an important factor led to Jill's newfound success as a scientist.
The title text addresses a common criticism in scientific circles that science is only good if it has rigor, that is if it is well documented and follows all of the proper procedure. It says that if curiosity without rigor is bad (in other words someone earnestly trying to figure out the answer, but doing it in a sloppy way) the opposite or rigor without curiosity is much worse (a person who produces professional looking results but who doesn't care about if they are right or wrong). Randall has previously suggested that rigor is not as important in science as some make it out to be, when discussing MythBusters (see 397: Unscientific).
Transcript
| This is one of 41 incomplete transcripts: Don't remove this notice too soon. If you can fix this issue, edit the page! |
- [Miss Lenhart is standing in front of a whiteboard with some scribbles on it.]
- Miss Lenhart: I'm supposed to give you the tools to do good science.
- [Miss Lenhart is now standing in front of Jill and Cueball, who are seated at classroom desks.]
- Miss Lenhart: But what are those tools?
- Miss Lenhart: Methodology is hard and there are so many ways to get incorrect results.
- Miss Lenhart: What is the magic ingredient that makes for good science?
- [Miss Lenhart headshot.]
- Miss Lenhart: To figure it out, I ran a regression with all the factors people say are important:
- [A list, presented in a sub-panel that Miss Lenhart is pointing to:]
- Outcome variable:
- • correct scientific results
- Predictors:
- • collaboration
- • skepticism of others' claims
- • questioning your own beliefs
- • trying to falsify hypotheses
- • checking citations
- • statistical rigor
- • blinded analysis
- • financial disclosure
- • open data
- [presumably the list goes on, as it runs off the visible part of the panel]
- [Another Miss Lenhart headshot.]
- Miss Lenhart: The regression says two ingredients are the most crucial:
- 1) genuine curiosity about the answer to a question, and
- 2) ammonium hydroxide
- [Miss Lenhart, standing, and Jill, seated at desk]
- Jill: Wait, why did ammonia score so high? How did it even get on the list?
- Miss Lenhart: ...and now you're doing good science!
Discussion
Should I find it comforting that, faced with "ammonium hydroxide", the student(?) decodes that as basically just "ammonia"? I mean, there are differences between anhydrous and hydrated versions, but it implies a certain amount of relevent scientific knowledge. If I mentioned "whateverium phlobotomide", the uninitiated would (as well as maybe stumbling over any unfamiliar parts of the name) probably be blind to it potentially being just a technical variation of the essential part of the name. Or, to put it another way, the technobable involved if you were to be told that all the dangerous dihydrogen monoxide had been swapped out for hydrogen hydroxide. (Or that it had been even more dangerous by introducing some trace amounts of nullanol.) 92.23.2.228 22:49, 11 June 2025 (UTC)
- When I studied chemistry, you would get an earful from the professor if you ever dared to utter the phrase "ammonium hydroxide", because ammonium hydroxide does not exist. It's not a molecule, and there's no crystalline ammonium hydroxide, either; what you have is aqueous ammonia, a small fraction of which gets protonated by water, forming some ammonium ions and some hydroxide ions. Calling that ammonium hydroxide would be akin to calling aqueous acetic acid "hydronium acetate". Perhaps that's why ammonia is in italics in the comic: to emphasize that the reply is meant as a correction. --Itub (talk) 11:03, 12 June 2025 (UTC)
I'm thinking that there are two uses for rigour without curiosity. The explanation covers the professional motive: to have a career in producing good-looking results. There is also a political motive: to block a line of research, to prevent its results from gaining traction. Table 1 of this: [1] covers a variety of kinds of sociology, most of which could have opponents. 112.213.42.56 02:06, 12 June 2025 (UTC)
Hi, I think there's an alternative reading for this one in the last panel, the first time I read it as dismissing the student's question and continuing her statement, which is a quite common move in other comics and always implied with ....... This way, the research became a meta-critique of bad science and somewhat links to rigor vs curiosity. First time commenting here, idk what to do now. 2A11:3:200:0:0:0:0:2004 03:21, 12 June 2025 (UTC)?
Miss Lenhart has made the mistake common among many scientists of failing to include repeatability among her factors to be tested. Had she done so, ammonia would have disappeared from the results. 82.13.184.33 08:28, 12 June 2025 (UTC)
- It's on the list, right below "adequate sample size" and "whether the lab has a lucky mascot" 185.177.139.23 10:00, 12 June 2025 (UTC)
I think there's a potential spin on the title text that I don't currently see addressed, but it might be in line with Randall's seeming attitude towards such matters: Rigour without curiosity doesn't need to be *wrong*, it's just that it's *joyless*. Or maybe *soulless*? The idea being that if you do rigorously correct but completely incurious science, you're doing it wrong even if the results themselves are technically correct. (Which they probably would be, given all that rigour.) Viewed this way, it's a critique of a particular kind of science that often comes in for criticism: endless variations on some (seemingly) unimportant experimental theme, for example, might be viewed as a way to generate publications without generating any actual advancement of science. 45.74.123.106 (talk) 19:07, 12 June 2025 (please sign your comments with ~~~~)
I'd argue that the student's response isn't only demonstrating good science by way of skepticism, as the current explanation suggests, but in fact demonstrating the first 5 factors all at once! Collaborating in the lesson, rather than it being purely a lecture, skepticism of other's claims (as mentioned already), questioning her own beliefs (is ammonia more important in science than I realized?), trying to falsify hypothesis (the hypothesis that ammonia is essential in science, in this case), checking citations (wait, what is this claim based on?). Plenty of good science being done! PotatoGod (talk) 16:18, 13 June 2025 (UTC)